VENCLEXTA +
azacitidine
was
proven
to help some
adults with
AML live longer
Actor portrayal.
Learn about treatment with VENCLEXTA + azacitidine
In a clinical study, VENCLEXTA + azacitidine was found to help some adults with AML live longer than those treated with azacitidine alone.
VENCLEXTA + azacitidine was studied in 431 adults with newly diagnosed AML who were age 75 years or older, or who had other medical conditions that prevented the use of standard chemotherapy. In the study, 286 patients received VENCLEXTA + azacitidine and 145 patients received azacitidine + placebo (an inactive medication), also considered as azacitidine alone.
Median overall survival
Half of patients treated with VENCLEXTA + azacitidine were still alive at 15 months vs 10 months for those receiving treatment with azacitidine alone.


VENCLEXTA may not work for everyone.
VENCLEXTA can cause serious side effects, including tumor lysis syndrome, low white blood cell count, and infections. These are not all of the possible side effects of VENCLEXTA. Talk to your healthcare provider for more information about the risks and side effects of VENCLEXTA. Please see additional important safety information below.
Median overall survival refers to the length of time from the start of treatment in a clinical trial to the time that half of the patients are still alive.
Median means the middle number in a group of numbers that are arranged from lowest to highest. For example, in the group of numbers 1 to 13, 7 is the median.
Actor portrayal.
Remission may be possible with VENCLEXTA + azacitidine
More patients treated with VENCLEXTA + azacitidine achieved remissions than with azacitidine alone.
When treated with VENCLEXTA + azacitidine
Percentage of patients achieving complete remission (CR) and complete remission with partial hematologic recovery (CRh)
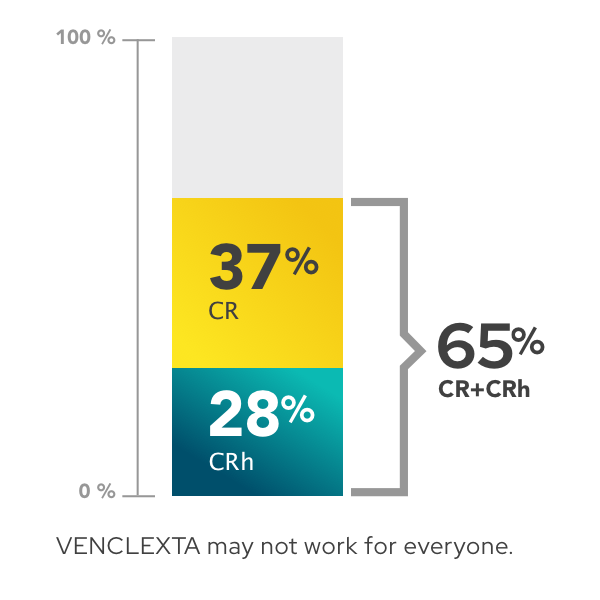
- 37% (105 of 286 people) achieved complete remission (CR)
- 28% (80 of 286 people) achieved complete remission with partial hematologic recovery (CRh)
- 65% (185 of 286 people) achieved complete remission (CR) or complete remission with partial hematologic recovery (CRh)
When treated with azacitidine alone
Percentage of patients achieving complete remission (CR) and complete remission with partial hematologic recovery (CRh)
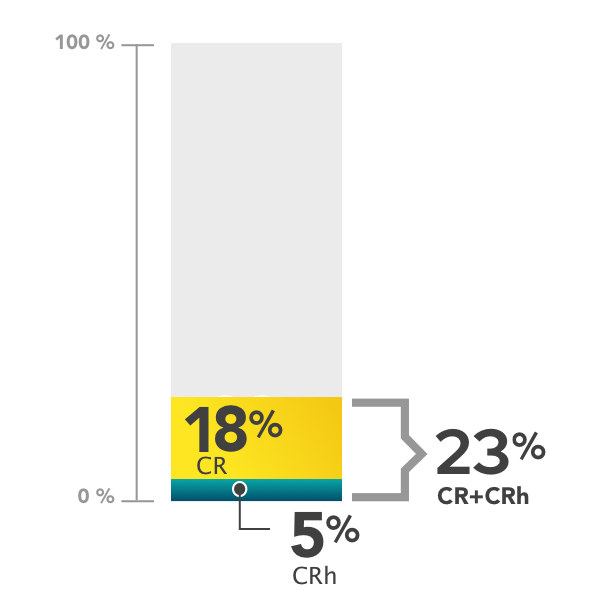
- 18% (26 of 145 people) achieved complete remission (CR)
- 5% (7 of 145 people) achieved complete remission with partial hematologic recovery (CRh)
- 23% (33 of 145 people) achieved complete remission (CR) or complete remission with partial hematologic recovery (CRh)
How long does remission last?
For patients treated with VENCLEXTA + azacitidine, the median length of time spent in:
- complete remission (CR) was 18 months compared to 13 months for patients treated with azacitidine alone
- complete remission (CR) + complete remission with partial hematologic recovery (CRh) was 18 months compared to 14 months for patients treated with azacitidine alone
Remission means a decrease in or disappearance of signs and symptoms of cancer.
CR, or complete remission, means the blood count is normal, fewer than 5% of bone marrow cells are leukemia cells, and there are no signs or symptoms of leukemia elsewhere in the body. When there is CR:
- Patients do not need to receive red blood cell transfusions
- Patients may not need to receive platelet transfusions based on their platelet levels and no signs of bleeding
CRh, or complete remission with partial hematologic recovery, means that some remission has occurred. When there is CRh, no signs of cancer are seen, but some blood counts have not returned to normal levels.
Median means the middle number in a group of numbers that are arranged from lowest to highest. For example, in the group of numbers 1 to 13, 7 is the median.
Side effects may occur with VENCLEXTA + azacitidine.
How long does it take for patients to respond to treatment?
In the clinical study, the median time for patients to achieve some level of remission for either CR or CRh was 1 month (with a range of 0.6 to 14.3 months). Remember, VENCLEXTA works differently for everyone. The time it takes to work and how long the effects last may vary from person to person.
Median means the middle number in a group of numbers that are arranged from lowest to highest. For example, in the group of numbers 1 to 13, 7 is the median.




