Sign up for more info on VENCLEXTA,
including tools and resources to help
you
make an informed treatment decision.
VENCLEXTA + GAZYVA®
(obinutuzumab)
dosing schedule
Actor portrayal.
Treatment with VENCLEXTA + GAZYVA is designed to be completed in 12 months
VENCLEXTA is a pill that you take once a day. GAZYVA, an antibody infusion therapy, is the beginning of the VENCLEXTA + GAZYVA regimen.
You will start taking VENCLEXTA at a low dose and gradually build up to a higher dose—this is known as a “ramp-up” period. This dose ramp-up is designed to gradually destroy your cancer cells and may help reduce your risk of tumor lysis syndrome (TLS).
Your healthcare provider may tell you to take VENCLEXTA differently than described here. Take VENCLEXTA exactly as prescribed by your healthcare provider. Do not start taking VENCLEXTA until you have reviewed the specific instructions with your healthcare provider. You may be directed to take your first dose and any increase in dose in your healthcare provider’s office or at the hospital.
How should VENCLEXTA be taken?
- Do not change your dose of VENCLEXTA or stop taking VENCLEXTA unless your healthcare provider tells you to
- When you first take VENCLEXTA, you may need to take VENCLEXTA at a hospital or clinic to be monitored for TLS
- Take VENCLEXTA 1 time a day with a meal and water at about the same time each day
- Swallow VENCLEXTA tablets whole. Do not chew, crush, or break the tablets
- If you miss a dose of VENCLEXTA and it has been less than 8 hours, take your dose as soon as possible. If you miss a dose of VENCLEXTA and it has been more than 8 hours, skip the missed dose and take the next dose at your usual time
- If you vomit after taking VENCLEXTA, do not take an extra dose. Take the next dose at your usual time the next day
What is the most important information to know about VENCLEXTA?
- Drink plenty of water during treatment with VENCLEXTA to help reduce your risk of getting TLS
- Drink 6 to 8 glasses (about 56 ounces total) of water each day, starting 2 days before your first dose, on the day of your first dose of VENCLEXTA, and each time your dose is increased
- Your healthcare provider may delay, decrease your dose, or stop treatment with VENCLEXTA if you have side effects
See important information about getting started with VENCLEXTA.
Month 1
Weeks 1–3: In the first month, you will receive GAZYVA on the first day of weeks 1–3; however, your very first dose of GAZYVA will be split over days 1 and 2.
Week 4: After the first 3 weeks of GAZYVA treatment, you will start taking VENCLEXTA once a day at a low dose and gradually build up to a higher dose—this is known as a ramp-up period.
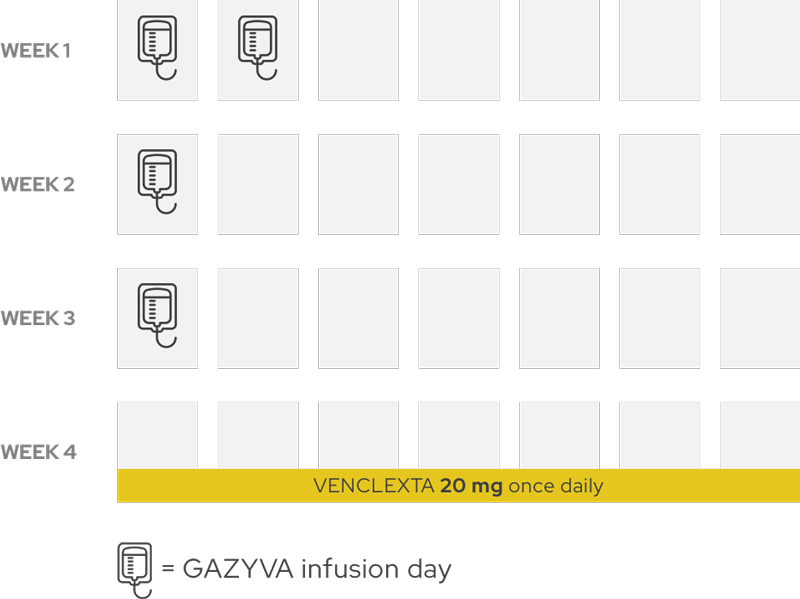
Month 2
Throughout the second month, you will gradually build up to a higher dose of VENCLEXTA and receive GAZYVA once during the month.
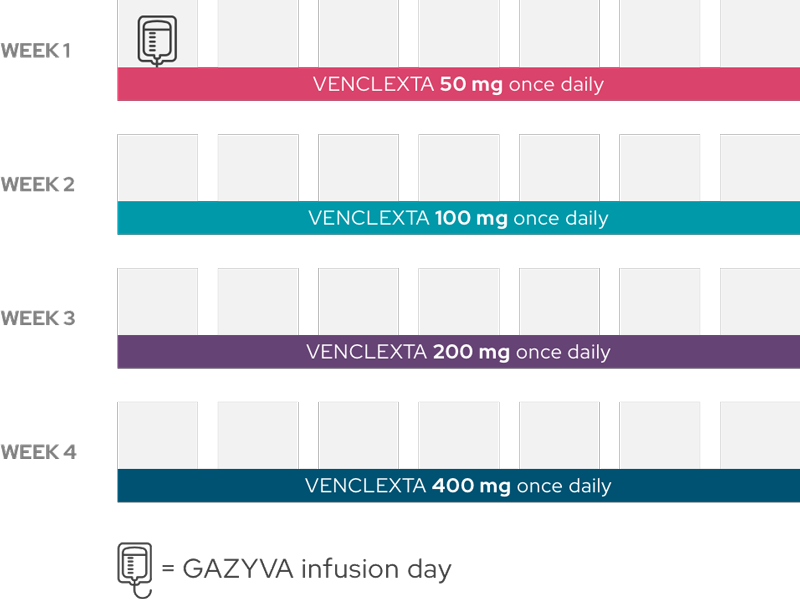
Months 3–6
You will receive GAZYVA once during the month, on the first day of the month and continue to take VENCLEXTA once daily for months 2–6.
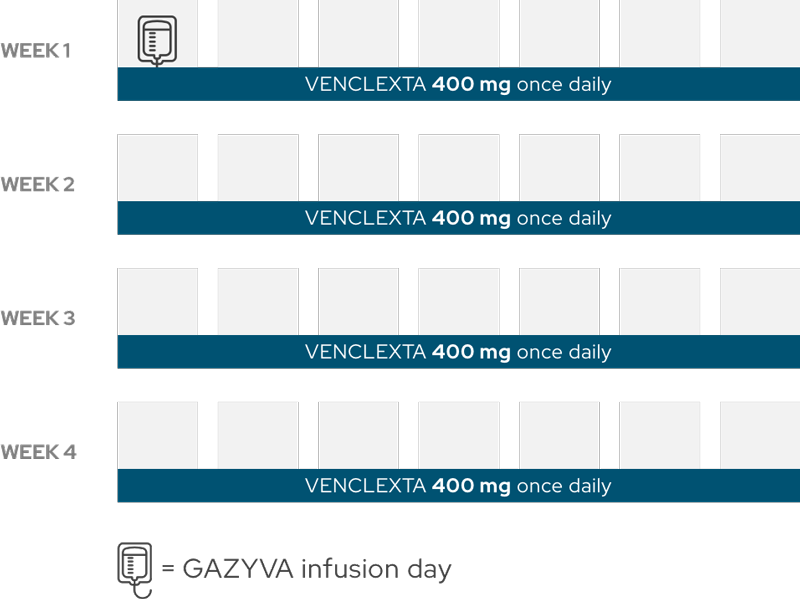
Months 7–12
You will continue to take VENCLEXTA once daily until the end of the 12-month treatment period. VENCLEXTA should be taken at the recommended daily dose as prescribed until your healthcare provider tells you to stop taking VENCLEXTA or changes your dose.

PATIENT BROCHURE
Get more information about treatment with VENCLEXTA

It is not known if VENCLEXTA is safe and effective in children.
Actor portrayal.
Learn more about VENCLEXTA treatment


Sign up for more info on VENCLEXTA,
including tools and resources to help
you make an informed treatment decision.



