Normal Cell
When normal cells are old or damaged, your body triggers them to self‑destruct. This process is called apoptosis (ay‑pop‑toh‑sis).
Get VENCLEXTA treatment support: (844) 926-6727, M–F, 7am–7pm CST | Sign up for THE VEN ZONE
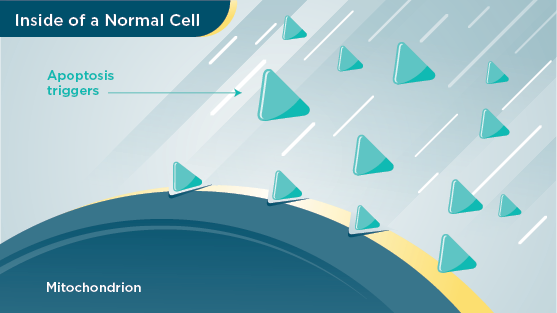
Normal Cell
When normal cells are old or damaged, your body triggers them to self‑destruct. This process is called apoptosis (ay‑pop‑toh‑sis).
Your healthcare provider may prescribe VENCLEXTA with an intravenously administered antibody therapy, such as GAZYVA® (obinutuzumab) or rituximab, to help you fight CLL or SLL.
GAZYVA and rituximab are a type of antibody therapy that targets and attaches to the CD20 protein found on the surface of CLL cells as well as some healthy blood cells. Once attached to the CD20 protein, GAZYVA and/or rituximab is thought to work in different ways, such as:
VENCLEXTA is a prescription medicine used to treat adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
It is not known if VENCLEXTA is safe and effective in children.
VENCLEXTA can cause serious side effects, including:
Tumor lysis syndrome (TLS). TLS is caused by the fast breakdown of cancer cells. TLS can cause kidney failure, the need for dialysis treatment, and may lead to death. Your healthcare provider will do tests to check your risk of getting TLS before you start taking VENCLEXTA. You will receive other medicines before starting and during treatment with VENCLEXTA to help reduce your risk of TLS. You may also need to receive intravenous (IV) fluids into your vein. Your healthcare provider will do blood tests to check for TLS when you first start treatment and during treatment with VENCLEXTA. It is important to keep your appointments for blood tests. Tell your healthcare provider right away if you have any symptoms of TLS during treatment with VENCLEXTA, including fever, chills, nausea, vomiting, confusion, shortness of breath, seizures, irregular heartbeat, dark or cloudy urine, unusual tiredness, or muscle or joint pain.
Drink plenty of water during treatment with VENCLEXTA to help reduce your risk of getting TLS. Drink 6 to 8 glasses (about 56 ounces total) of water each day, starting 2 days before your first dose, on the day of your first dose of VENCLEXTA, and each time your dose is increased.
Your healthcare provider may delay, decrease your dose, or stop treatment with VENCLEXTA if you have side effects. When restarting VENCLEXTA after stopping for 1 week or longer, your healthcare provider may again check for your risk of TLS and change your dose.
Certain medicines must not be taken when you first start taking VENCLEXTA and while your dose is being slowly increased because of the risk of increased TLS.
Before taking VENCLEXTA, tell your healthcare provider about all of your medical conditions, including if you:
You should not drink grapefruit juice or eat grapefruit, Seville oranges (often used in marmalades), or starfruit while you are taking VENCLEXTA. These products may increase the amount of VENCLEXTA in your blood.
VENCLEXTA can cause serious side effects, including:
Tell your healthcare provider right away if you have a fever or any signs of an infection during treatment with VENCLEXTA.
The most common side effects of VENCLEXTA when used in combination with obinutuzumab or rituximab or alone in people with CLL or SLL include low white blood cell counts; low platelet counts; low red blood cell counts; diarrhea; nausea; upper respiratory tract infection; cough; muscle and joint pain; tiredness; and swelling of your arms, legs, hands, and feet.
VENCLEXTA may cause fertility problems in males. This may affect your ability to father a child. Talk to your healthcare provider if you have concerns about fertility.
These are not all the possible side effects of VENCLEXTA. For more information, ask your healthcare provider or pharmacist.
You are encouraged to report side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1‑800‑FDA‑1088.
If you cannot afford your medication, contact genentech-access.com/patient/brands/venclexta for assistance.
US-VENC-210314
Please see full Prescribing Information, including Medication Guide.